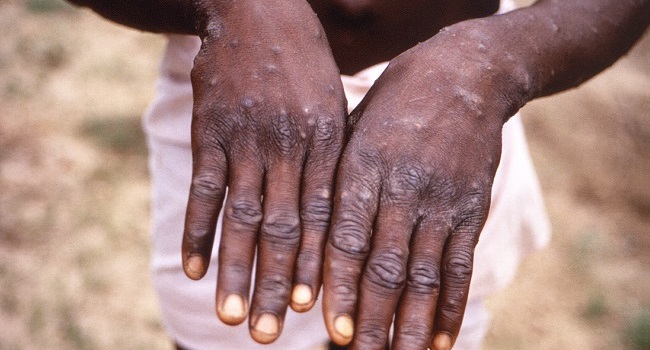
The antiviral drug tecovirimat (TPOXX) could become ineffective against the monkeypox virus as a result of overuse, US health officials have warned. Tecovirimat is currently the only drug that is available and approved to treat monkeypox. It is feared that misuse could trigger mutation of the virus that could render the pills ineffective.
The Food and Drug Administration, FDA, recently updated its guidance for Tpoxx which is being prescribed to patients with the monkeypox virus.
In an online update, FDA officials cautioned that a single molecular change to monkeypox “could have a large impact on the antiviral activity of Tpoxx.” Noting that viruses are constantly evolving to overcome obstacles to infection, including drugs, regulators urged doctors to be judicious in prescribing the medication.
In a related event, the Centers for Disease Control and Prevention, CDC, says Tpoxx should no longer be given to otherwise healthy adults who are not suffering severe symptoms.
Doctors wishing to prescribe the drug must submit an application to CDC, documenting their patient’s need and agreeing to track their results and any side effects. Officials have shipped 37,000 courses of the drug to physicians.
Tpoxx works by targeting a single protein found on monkeypox, smallpox and similar viruses. The FDA said this week that research in labs, animals and people suggests multiple ways in which monkeypox could develop resistance to the therapy.
The FDA approved the medication in 2018 under its “animal rule,” which allows approval based on animal data when human testing is unethical or unfeasible. Smallpox was declared eradicated in 1980 by the World Health Organization, ruling out the possibility for human studies.
The CDC reported last week that 3.5 percent of patients tracked through its Tpoxx programme reported side effects, mainly headache and nausea.
The agency has only gotten back about 200 forms from physicians documenting patient’s initial symptoms and results, accounting for less than 1 percent of the doses shipped since the start of the outbreak.